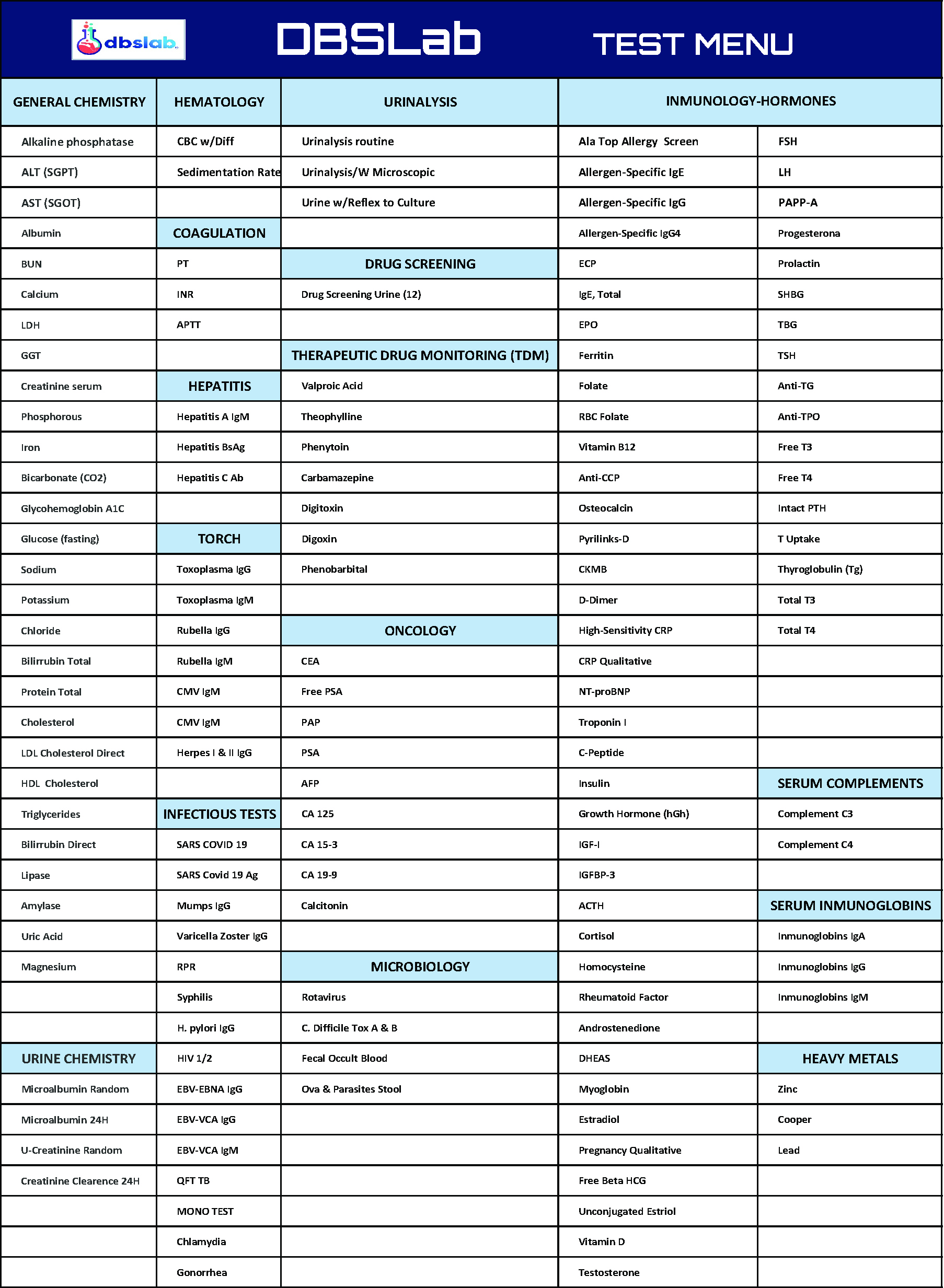
| Standard Safety & Chemistry |
Standard hematology, biochemistry panels, urinalysis, serology, and coagulation testing. |
| Molecular Biology |
(q)PCR, RT-(q)PCR, dd-PCR, Multiplex PCR, BioFire FilmArray, and Seegene panels. |
| Lab Testing in Clinical Trials |
Customized biomarker-driven trial solutions, including full assay development and technology transfer. |
| Flow Cytometry |
High-parameter analysis using BD FACS Canto™ II (12-Color), Cytek Aurora (>40 Colors), and BD FACS Lyric (for whole blood, bone marrow, and PBMC). |
| Histopathology & Anatomic Pathology |
Multiplex and standard IHC, Immunostaining, ISH/FISH, 200+ biomarkers. Expertise in FFPE and Cryosections using Leica and Roche Ventana (RUO/IVD). |
| Genetics & NGS |
NGS (WES, WGS), mutation analysis, Illumina short-read sequencing (MiSeq, HiSeq, NextSeq, NovaSeq), PacBio and Oxford Nanopore Technologies long-read sequencing, Sanger sequencing, and Nanostring analysis. |
| Cell and Gene Therapy |
Up to 4th-field HLA typing, minimal residual disease (MRD), Replication Competent Lentivirus/Retrovirus testing (RCL/RCR), Vector Copy Number (VCN), Vector Integration Site analysis (VIS/ISA), therapeutic vector copies, shedding studies (PK/PD), immune repertoire sequencing, TAbs/ADA for vectors/proteins, and viral vector sequence integrity. |
| Microbiology |
Culture and identification, antibiotic resistance testing, microbiome analysis (sequencing), targeted sequencing, bacterial whole-genome sequencing (including hybrid assemblies), and whole metagenome sequencing. |
| Cell Isolations |
In-house isolation of PBMC, BMMC, and CD138+ cells, supported by a network of pre-analytical processing labs. |
| ELISA & Multiplex Assays |
ELISA, MSD S600 Meso Scale Discovery, Luminex, Ella ProteinSimple, and Quanterix Simoa HD1. |
| Bioanalysis (PK/PD) |
Pharmacokinetics (PK), Pharmacodynamics (PD), Anti-Drug Antibodies (ADA), small & large molecule analysis, and ELISA-based methods using LC-MS/MS. |
| Functional Cell-Based Assays |
Specialized assays including T-cell activation, ADCC (Antibody-Dependent Cell-mediated Cytotoxicity), and ELISpot. |
| Virology Testing |
Virus Culture, TCID50, neutralization assays, inhibition assays, viral load, genotyping & phenotyping, short and long-read sequencing, minor variant analysis, antiviral resistance mutations, consensus sequencing, and Virome analysis. |
| Digital Imaging & Analysis |
Transforming visual results into quantifiable data using state-of-the-art Halo® and Visiopharm® Software. |
| Bioinformatics Solutions |
Customized pipelines in virology and bacteriology, reference conversion options, and tailor-made analysis of clinical trial data (e.g., treatment emerging mutations). |